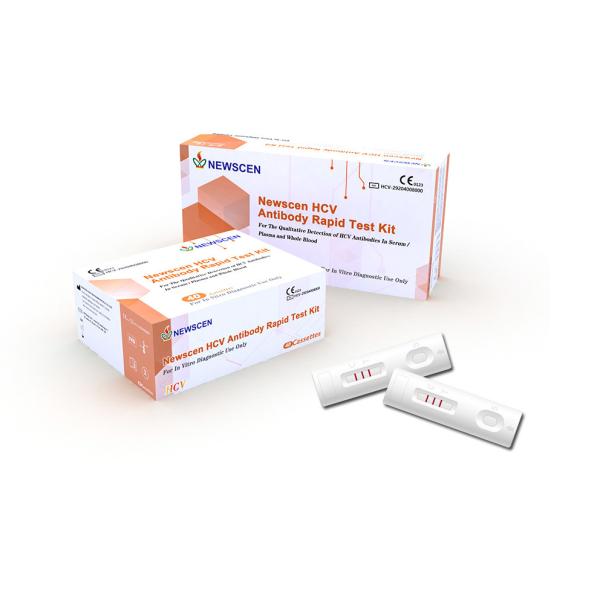
HCV(Hepatitis C Virus) Antibody Rapid Test Kit
For the Qualitative Detection of HCV Antibodies in serum/plasma and
whole Blood
Main Features
☀ High Sensitivity
☀ High Specificity
☀ Simple: No Instrument Required
☀ Reliable: high accurate, early detection of the presence of HCV
☀ Convenient: Room Temperature Storage, Built-In Control line
☀ Fast: Results in 5-30 minutes, strong positive results may be
observed promptly within 3 minutes
☀ Certified by Authoritative Certification System and Standards
☀ Winner of "the National Rapid Diagnostic Kit for Clinical
Performance Assessment"

Intended Use
Newscen HCV Antibody Rapid Test is for the detection of antibodies
to Hepatitis C type virus(HCV) in human serum, plasma or whole
blood collected from vein or fingertip. It is intended for use in
medical institutions by trained staff.
Principle
HCV(Core and NS 3) specific recombinant antigens are precoated on
to the membrane as the capture reagent on the test zone. During the
test, specimen is allowed to react with the colloidal gold
particles,which have been conjugated with HCV specific recombinant
antigens. Antibodies to HCV, if present, will specifically bind to
colloidal gold-antigen complex.
When the colloidal gold-antigen-antibody complexes move to the test
zone, they will specifically bind to the precoated antigens. At the
same time, a red colored Iine will develop in the test zone.
Absence of this red colored line in the test zone suggests a
negative result. To serve as a procedural control, red colored line
in the control zone will always appear regardless of the presence
of antibodies to HCV.

Materials Required But Not Provided
☀ Timer or stopwatch
☀ Blood collection devices, for the testing of venipuncture whole
blood, serum or plasma
☀ Biohazard disposal container
☀ Disposable gloves
For finger stick samples, the following materials are required:
Alcohol pad
Sterile lancet
Sterile gauze or cotton
Reagents and Materials Provided
Each kit contains:
☀ 40 test cassettes(individually pouched)
☀ Each pouch contains one cassette with one desiccant bag
☀ One bottle of diluent buffer(5mL)
☀ 40 disposable plastic droppers
☀ Instruction for use
Assay Procedure
☀ Place the test cassette on flat surface. Before unseal the pouch,
allow the test cassette to reach laboratory temperature(15-30°C)
.Use it immediately once unsealed.
☀ Open the pouch and add1dropor35pL of specimen into the sample
well(S) .
☀ After adding the specimen, add1dropor35pL of diluent buffer
vertically into the sample well(s) immediately.
☀ Avoid dropping specimen or diluent buffer in the observation
window.
☀ Do not allow the diluent buffer bottle touch the sample well when
dropping the diluent buffer so as to prevent the cross
contamination with the specimen.
☀ Observe the result in 30 minutes once the diluent buffer added.
Interpretation of Results

Note:'C'-Control line; T'-Test line
Negative: No redline appear in 30 minutes in the test zone(T), only a
redline in the control zone(C) ,which indicates that the absence of
HCV antibodies or the concentration of antibodies is too low(i.e.,
window period of infection) .
Positive: One redline in the control zone(C) and one redline in the test
zone(T) .This indicates the specimen contains HCV antibodies.
Invalid: If no redlines appear, the test is invalid. Discard the test
cassette and perform with new cassette.

Built-In Control
Newscen HCV Antibody Rapid Test has a built-in procedural control
that demonstrates assay validity. A redline appeared on the control
zone(C) indicates that the test runs correctly.
Warning
For invitro diagnostic use ONLY
Storage
Newscen HCV Antibody Rapid Test should be stored at 4-30°C(do not
freeze) for 24 months from the date of manufacture. Keep the test
cassette in sealed pouch until use. Once you have taken the test
cassette out of the pouch, perform the test as early as
possible(within 1hour) to avoid test cassette from becoming moist.
Do not use the test beyond the indicated expiration date.
The diluent buffer should be stored at 4-30°C(do not freeze) for 24
months from the date of manufacture. After the diluent is opened,
it will be stored for 24 months according to the validity period.